Instructions for disposable sterile gastroscopy
[product name]: disposable aseptic gastroscopy package
[specification / model]: type A and type B
[name of registrant]: Xinxiang Hongda sanitary materials Co., Ltd
Address: dingluan Industrial Zone, Changyuan County, Henan Province
[contact information]: Tel: 0373-8996070 Fax: 0373-8996966
[after sales service unit]: Xinxiang Hongda sanitary materials Co., Ltd
[name of manufacturer]: Xinxiang Hongda sanitary materials Co., Ltd
Address: dingluan Industrial Zone, Changyuan County, Henan Province
[registered address]: dingluan Industrial Zone, Changyuan County, Henan Province
[production address]: dingluan Industrial Zone, Changyuan County, Henan Province
[contact information]: Tel: 0373-8996070 Fax: 0373-8996966
Email: xxshd@126.com Postcode: 453412 website: www.kaiquan.cc
[license No.]: ysyjx production license No. 20150111
[Registration Certificate No.: yxzz 20162660527
[registered product standard]: yzb / Yu 0717-2014
[production date]: see the label of the certificate or the outer packing bag
[production batch No.]: see certificate label or outer packing bag
[expiration date]: see certificate label or outer packing bag
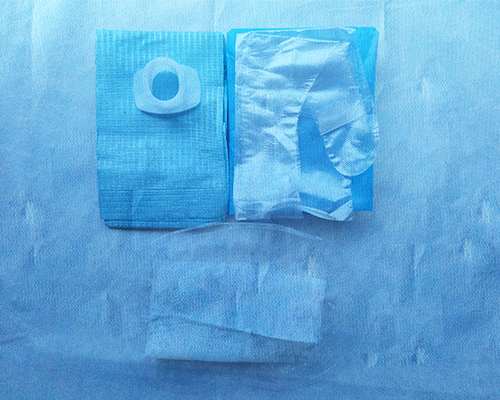
[main structure and performance]: the product is composed of basic configuration: disposable film inspection gloves, disposable medical wrapping cloth and optional configuration: tray, disposable medical bite, tissue paper, tweezers, medical cotton ball and medical cotton swab. The product should be sterile. After sterilization with ethylene oxide, the residual amount of ethylene oxide should not be more than 10 μ g / g. Disposable use.
Scope of application: it is used for gastroscopy in clinic.
[usage]: open the package and use it directly.
[precautions]: 1. This product is sterilized with ethylene oxide, and its aseptic validity period is two years;
2. Check the validity period of sterilization before use and unseal with sterile scissors;
3. Do not use if damaged;
4. This product is disposable and destroyed after use.
[storage]: the product should be stored in a cool, dry and well ventilated warehouse with relative humidity no more than 80%, no corrosive gas.
[label and package identification] picture 2.png picture 3.png picture 4.png
Sterile ethylene oxide sterilization for single use
[preparation of instructions]
The instruction manual of this product is prepared and formulated in accordance with the provisions in the regulations on the management of instructions and labels of medical devices.